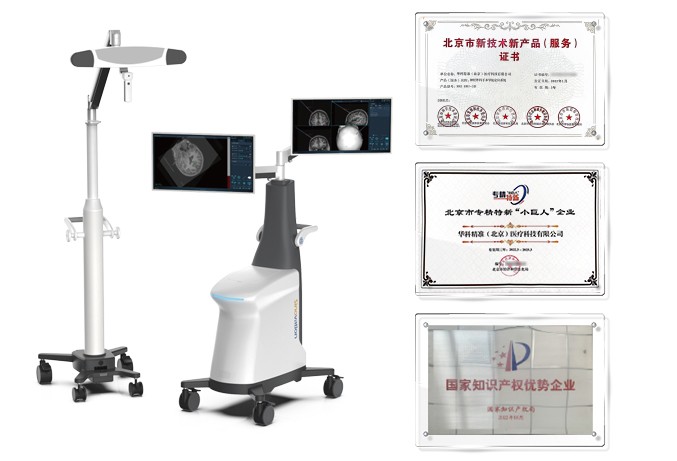
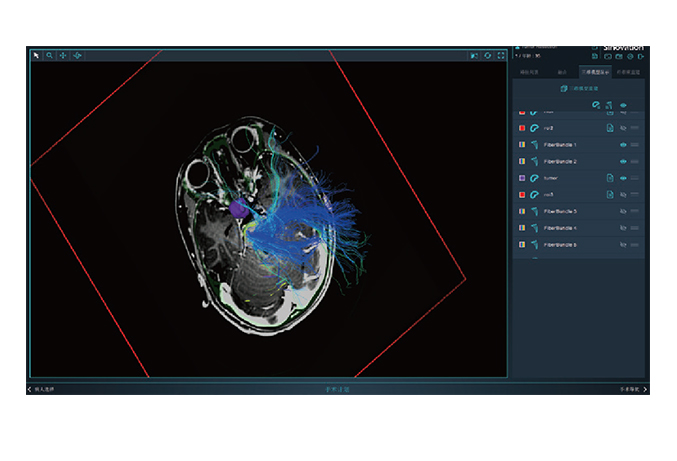
Sinovation Medical has independently developed nine products that have obtained Class III medical device registration certificates from the National Medical Products Administration (NMPA), with four of them approved through the special review channel for innovative medical devices. It has formed five major product lines, including the neurosurgical robot SR series, miniature neurosurgical robot Q300 series, neurosurgical navigation system NS series, intracranial electrode series, and magnetic resonance-guided laser ablation therapy system LS series. These products serve over 200 medical institutions nationwide, with the intracranial electrodes and neurosurgical robots ranking among the top in the domestic market share. Furthermore, the neurosurgical robot Sinobot X1 has also been appoved by the U.S. Food and Drug Administration (FDA), making it the only Chinese neurosurgical robotic system to obtain FDA approval.
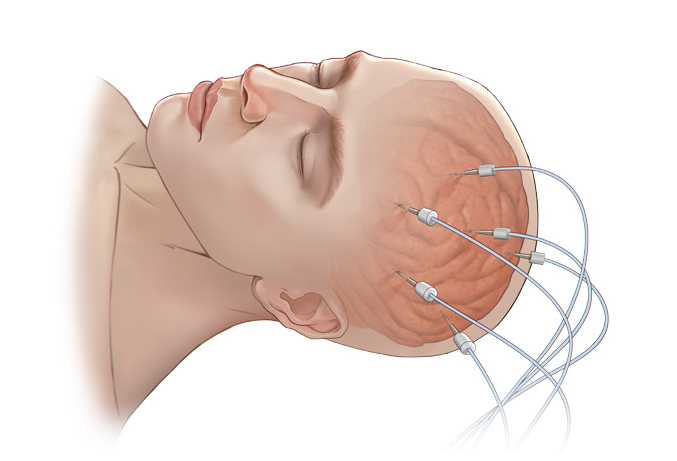
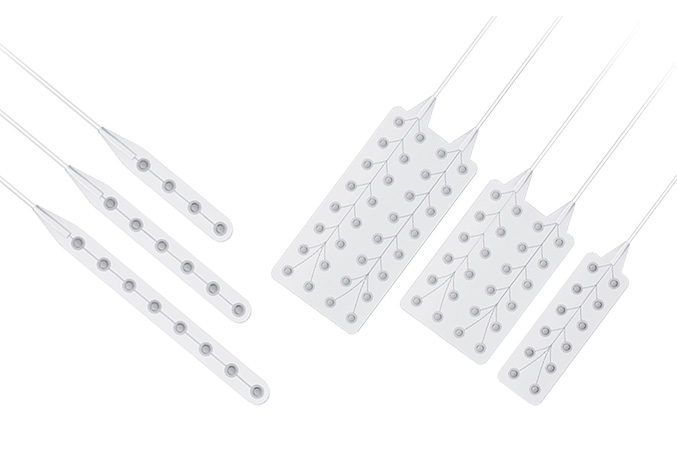
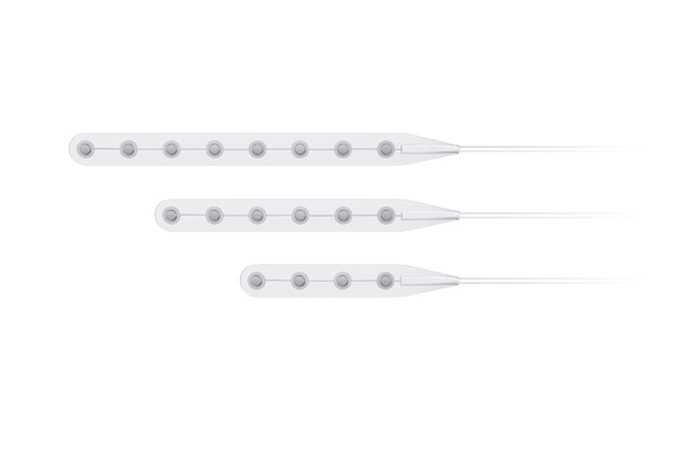
Its wholly-owned subsidiary, Beijing Sinovation Medical Technology Co., Ltd. (aka Huake Hengsheng Medical Technology Co., Ltd., HKHS), is a globally renowned manufacturer of intracranial electrodes. It specializes in the research and development, production, and technical services of medical electrodes in the field of neurosurgery. It is the first company in China to achieve commercialization of intracranial EEG signal acquisition consumables and is recognized as a national high-tech enterprise. The company has obtained four Class III medical device registration certificates and the annual sales volume of intracranial electrodes reaches tens of thousands. These electrodes are widely used in over 200 Class A tertiary hospitals nationwide, making HKHS a leader in the domestic market and successfully replacing imported products as a whole.
We not only focus on technological innovation but also prioritize providing high-quality solutions for the healthcare industry. Our aim is to enhance the level of medical diagnosis and treatment, thereby driving continuous progress in medical technology. Sinovation Medical, in collaboration with the healthcare industry, is dedicated to shaping the future of intelligent healthcare.